Establishment of The African Medicines Agency (Ama) is on Course
Establishment of The African Medicines Agency (Ama) is on Course

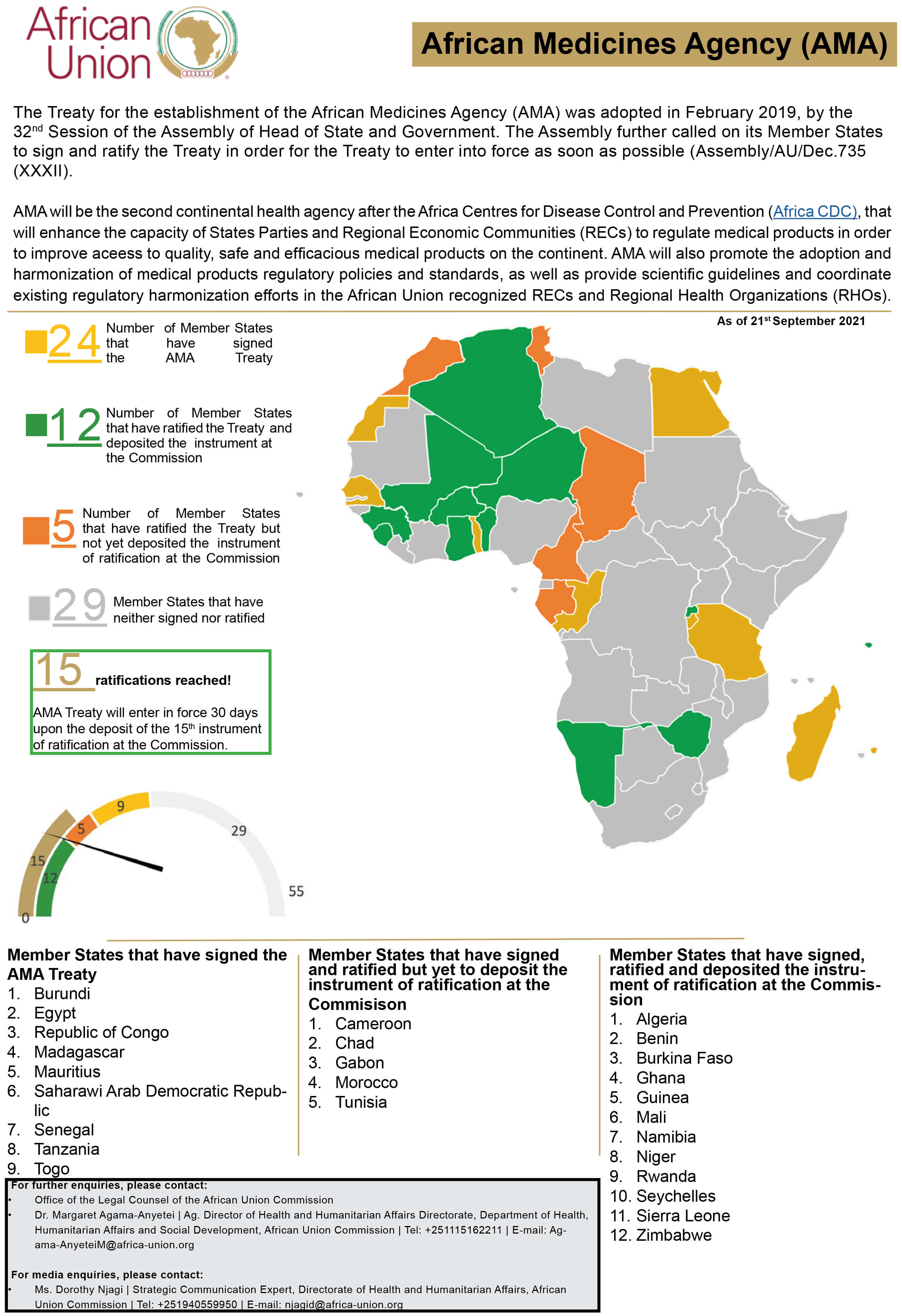
In February 2019, the Assembly of Heads of State and Government of the African Union (AU), adopted the legal instrument for the establishment of the African Medicines Agency (AMA) and called on its Member States, to sign and ratify the Treaty, in order for the Treaty to enter into force as soon as possible (Assembly/AU/Dec.735 (XXXII). The adoption of the Treaty for the establishment of AMA sets the Union on a course towards establishing a second continental health institution that shall significantly contribute to the improvement of healthcare delivery across the continent and better overall health of African citizens as envisioned in Africa’s Agenda 2063.
Following the establishment of the Africa Centres for Disease Control and Prevention (Africa CDC), the AMA will be the second specialised continental health agency established by the AU with its main function being to enhance the capacity of African Countries to regulate medical products, in order to, improve access to quality, safe and efficacious medicines, medical products and technologies on the continent.
The AMA is supported by AU programmes like the African Medicines Regulatory Harmonization (AMRH) which was started in 2009 as a response to addressing challenges faced by National Medicine Regulatory Authorities (NMRAs) in Africa such as weak or non-coherent legislative frameworks, redundant/duplicative processes, sluggish medicine registration processes and subsequent delayed decision, inefficiency and limited technical capacity, among others. AMRH aims to ensure that African people have access to essential medical products and technologies. The AMRH is implemented as part of the Pharmaceutical Manufacturing Plan for Africa (PMPA).
AMA will enter into Force thirty (30) days after the deposit of the fifteenth instrument of ratification of the AMA Treaty to the Commission.
As per Article 9 of the AMA Treaty, the AMA shall have its Headquarters in one of the AU members states, The Headquarters of AMA shall be determined by the Assembly upon the recommendations of State Parties.
Once the AMA Treaty is in Force the Commission shall convene the Conference of State Parties, which is the highest policy-making organ of the AMA composed of all Member States of the African Union who ratify or accede to AMA Treaty. The conference of State Parties will establish and determine the composition, sessions, functions and terms of office of the AMA Governing Board. The Governing Board will include 5 Heads of National Medicines Regulatory Authority (NMRAs) from each region, one (1) Regional Economic Community (REC) representative, one (1) representative of Regional Health Organization (RHOs), 1 representative of National Committees Responsible for bioethics on rotational basis and the Commissioner for Commissioner for Health, Humanitarian Affairs and Social Development (HHS) at the African Union Commission (AUC).
This will be followed by the appointment of the Director General by the Conference of the State Parties upon the recommendations of the Governing Board. The Director General of AMA will serve as the Head of AMA Secretariat and will be responsible for the day-to-day management of the AMA. Finally, the Secretariat of the AMA will be established and will be responsible for coordinating the implementation of the decisions of the conference of State Parties, the policy organs of AU and the Governing Board of AMA.
The Commission encourages all its Member States to sign and ratify the Treaty for the establishment of AMA, in the interest of public health, safety and security.
See the status of ratification on the accompanying infographic.